The Project Objectives
The projects objectives
What is LNG?
 Liquefied natural gas has been one of the most important energy sources worldwide for years. In order to enable the trade and transport of fossil natural gas over long distances, LNG terminals have been built around the world in recent decades to liquefy natural gas, load it onto tankers, vaporize it again in the destination ports, and feed it into the gas networks of the destination countries.
Liquefied natural gas has been one of the most important energy sources worldwide for years. In order to enable the trade and transport of fossil natural gas over long distances, LNG terminals have been built around the world in recent decades to liquefy natural gas, load it onto tankers, vaporize it again in the destination ports, and feed it into the gas networks of the destination countries.
Liquified Natural Gas
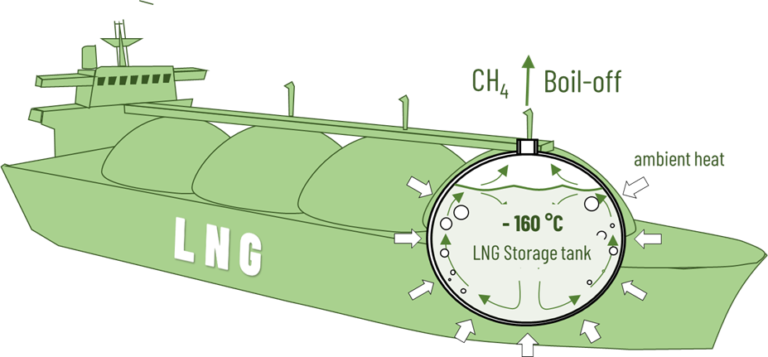 By cooling it to -160 °C, methane, the main component of natural gas, can be liquefied. Due to its high energy density, LNG can also be transported in large quantities across the oceans by tanker beyond the existing pipeline infrastructure. To avoid pressure build-up in the tanks due to heating during transport, it is accepted that a small part of the methane will vaporize. This boil-off is either liquefied or can be used for ship propulsion.
By cooling it to -160 °C, methane, the main component of natural gas, can be liquefied. Due to its high energy density, LNG can also be transported in large quantities across the oceans by tanker beyond the existing pipeline infrastructure. To avoid pressure build-up in the tanks due to heating during transport, it is accepted that a small part of the methane will vaporize. This boil-off is either liquefied or can be used for ship propulsion.
Existing and upcoming Markets for LNG
Electrification of the mobility sector will certainly continue to grow in the upcoming years. The transition to electric mobility based on renewables will have the potential to save more than 50% of the transport sectors actual carbon emissions. However, electrification comes to limitations where long-distance and heavy duty transportation requires fuels with high energy density. Current expectations for battery capacities suggest that a major part of the transportation sector – namely water navigation and aviation – will still depend on high energy density fuels for the next decades. LNG is already quite common for cruise vessels and will certainly be a preferred option for marine applications. The ongoing transition from fossil diesel and heavy oil fuel reduces pollutant and CO2 emissions, but the overall carbon emission of the water navigation sector will not significantly decrease due to the lack of renewable – truly carbon neutral – LNG.
Why LNG needs to be GreenLNG
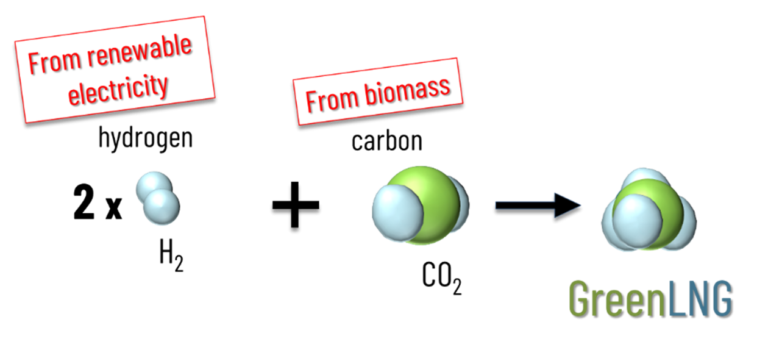 Currently, the LNG traded on the world markets comes exclusively from fossil natural gas. However, methane can also be synthesized from hydrogen and CO2. If the hydrogen comes from the electrolysis of water with electricity from renewable energies, the hydrogen carrier LNG can be used to store and transport renewable energy. However, for the synthesis of green LNG, it is not only important that the hydrogen for methanation comes from renewable sources, but also that the CO2 for the synthesis comes from the atmosphere. True green LNG is therefore only produced when the carbon comes from biogenic sources.
Currently, the LNG traded on the world markets comes exclusively from fossil natural gas. However, methane can also be synthesized from hydrogen and CO2. If the hydrogen comes from the electrolysis of water with electricity from renewable energies, the hydrogen carrier LNG can be used to store and transport renewable energy. However, for the synthesis of green LNG, it is not only important that the hydrogen for methanation comes from renewable sources, but also that the CO2 for the synthesis comes from the atmosphere. True green LNG is therefore only produced when the carbon comes from biogenic sources.
A key challenge: LNG transport and Methane emissions
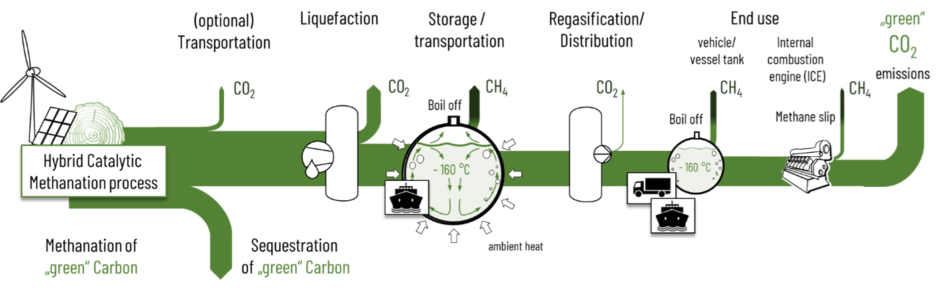
Similar to other process chains for transportation fuels the GreenLNG production suffers from upstream carbon emissions. Major carbon emissions result from energy consumption for the liquefaction. But the really crucial greenhouse gas emission are fugitive methane emissions from storage tanks, leakages of LNG infrastructures and the methane slip in internal combustion engines (Figure 6). Storage tanks store liquefied methane at minus 160 °C and ambient pressure. Ambient heat that passes the tank’s insulation evaporates small amounts of methane (“boil-off”) in order to keep the temperatures at -160 °C. These fugitive emissions are particularly harmful due to the high global warming potential (GWP20 = 83, GWP100 = 29,5) of methane in comparison to its combustion product CO2.
Our innovative appoach
The project proposes a new hybrid process chain for the synthesis of methane for liquefaction. The combination of chemical catalytic and biological catalytic processes uses the advantages of each technology to compensate for the disadvantages of the other technology. The proposed hybrid catalytic conversion process combines therefore
- thermochemical catalytic processes, i.e. carbon efficient sorption enhanced e-gasifier
- chemical catalytic processes, i.e. Catalytic Raw Methanation with in-situ tar hydrogenation
- biological catalytic processes, i.e. Biological Methanation with advanced biological process control
and
- bioelectrocatalytic excess carbon utilization by means of electromethanogenesis
